Bamlanivimab
« previous post | next post »
"Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibody for Treatment of COVID-19", U.S. Food and Drug Administration 11/9/2020:
Today, the U.S. Food and Drug Administration issued an emergency use authorization (EUA) for the investigational monoclonal antibody therapy bamlanivimab for the treatment of mild-to-moderate COVID-19 in adult and pediatric patients.
“Bamlanivimab”
Try saying that 5… or 9 times fast!
Jimmy tries to pronounce the new COVID antibody treatment https://t.co/Ua2rpQ0D0j #FallonMono #FallonTonight pic.twitter.com/LuDHpFuJt2
— The Tonight Show (@FallonTonight) November 11, 2020
So in IPA, the pronunciation is apparently something like
[ˌbæm.ləˈnɪ.vɪˌmæb]
| Audio |
But the pronunciation of bamlanivimab is just part of the puzzle — there's also the morphology.
From "GUIDANCE ON THE USE OF INTERNATIONAL NONPROPRIETARY NAMES (INNs) FOR PHARMACEUTICAL SUBSTANCES", WHO 2017:
The fact that there's a typo ("Sustem A") in this schema doesn't fill me with confidence.
From the cited source "International Nonproprietary Names (INN) for biological and biotechnological substances", WHO 2016:
- INN for monoclonal antibodies (mAbs) are composed of a prefix, a substem A, a substem B and a suffix.
- The common stem for mAbs is -mab, placed as a suffix.
- The stem -mab is to be used for all products containing an immunoglobulin variable domain which binds to a defined target.
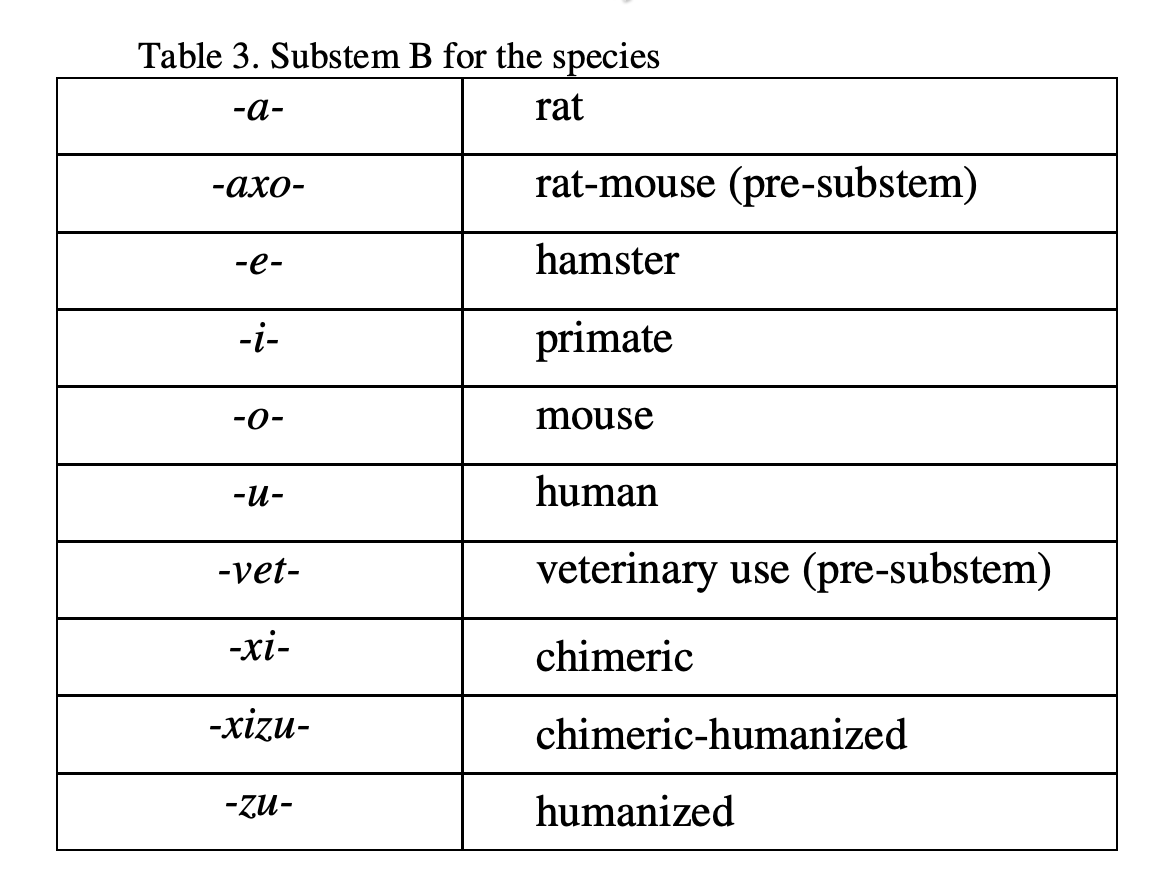
- Substem B indicates the species on which the immunoglobulin sequence of the mAb is based (shown in Table 3).
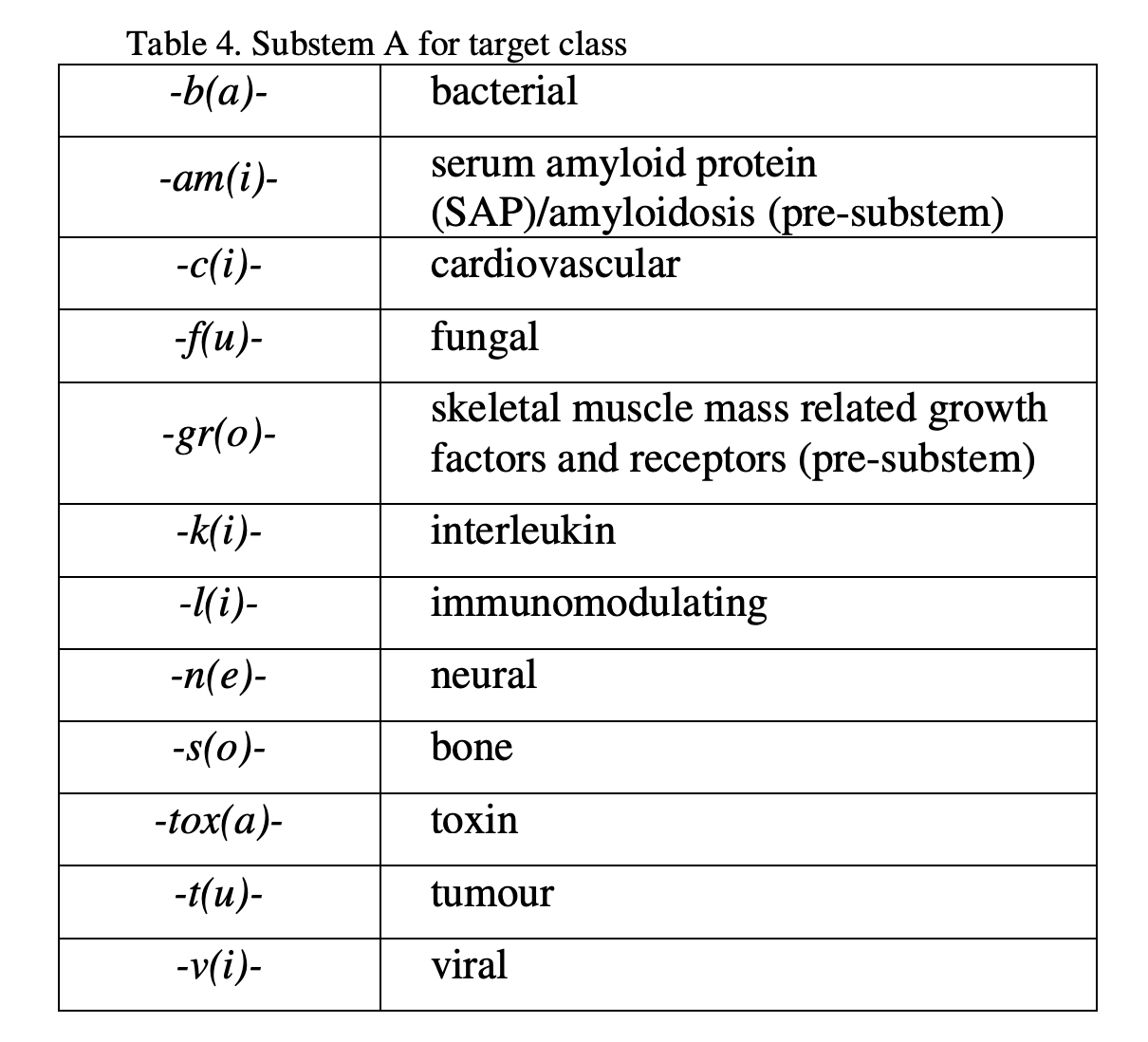
- Substem A indicates the target (molecule, cell, organ) class (shown in Table 4).
In principle, a single letter, e.g. -b- for bacterial is used as substem A. Whenever substem B starts with a consonant (e.g. x or z), to avoid problems in pronunciation, an additional vowel indicated in the table, e.g. -ba- is inserted.
The prefix should be random, i.e. the only requirement is to contribute to a euphonious and distinctive name.
OK, so how to parse bamlanivimab according to this scheme?
The final -mab is clear (though the WHO confusingly calls it both a stem and a suffix). But what about the initial bamlanivi- part?
Is the analysis this?
bamlani v i mab
PREFIX Substem A Substem B SUFFIX
"viral" "primate"
If so, was there any rhyme or reason behind the choice of "bamlani" as the prefix? Maybe fragments of the names of the developers?
And perhaps some reader can explain why the components of Regeneron's cocktail of two monoclonal antibodies, REGN-COV2, are named REGN10933 and REGN10987 rather than SomethingSomethingSomething-mab.
See also "Gotta catch 'em all", 11/20/2014; "New drug for medical burnout", 8/1/2019.
Update — it occurs to me that this is a concrete step in the direction of John Wilkins' Philosophical Language — the spirit of Jorge Luis Borges is no doubt mildly amused.


Ross Presser said,
November 13, 2020 @ 9:39 am
https://www.marketingweek.com/mark-ritson-bamlanivimab-brand-name-strategy/
"To make things even harder, the new name must not only avoid any reference to patient benefits but also to its basic usage too. It cannot even reference its initial intended medical application in the name…. bamlanivimab’s name is a product of this process. And to be fair, Eli Lilly did not even make the final decision to pick that name. Instead, it likely submitted a raft of proposed names and the FDA, the US government body in charge of drugs in America, made the final selection and then communicated it back to Eli Lilly."
Ross Presser said,
November 13, 2020 @ 9:42 am
https://www.idstewardship.com/5-things-know-bamlanivimab/
> Generic drug name bamlanivimab breaks down into “bamlan” (a two-syllable non-specific stem prefix meeting generic name requirements), “vi” (denotes it’s targeting a virus), and “mab” (monoclonal antibody). You can read all about naming monoclonal antibodies here, it’s actually pretty neat. I thought the first “i” in “bamlani” indicated the animal species it was derived from (human plasma in this case), but I can’t find anything to confirm it so have to leave that part open for now.
Ross Presser said,
November 13, 2020 @ 9:46 am
Finally, here's an updated version of that table:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6294595/table/T1/?report=objectonly
For post-2017 INN, there is no substem A and B, only one substem:
banlani – vi – mab
random prefix + viral + mab
VVOV said,
November 13, 2020 @ 9:54 am
Per Wikipedia, since 2017, “it was decided to drop the source substem” [Substem B, in the tables above] by the WHO subcommittee in charge of such things, because of “the difficulty in capturing the complexity and subtleties of the many methods by which antibody drugs can be produced.”
Thus, the morphology is simply bamlani + vi + mab.
I have always felt that a motivating factor for the opaque and near-unpronounceable naming of monoclonal antibodies is to force everyone – patients and professionals alike – to use the brand names out of convenience.
Ross Presser said,
November 13, 2020 @ 9:58 am
Finally-finally,
1) my last comment I miswrote "viral" as "virtual", sorry
2) The blahblahvimab names are *generic drug names* that follow this naming convention. REGN10933 and REGN10987 would therefore be not the final, official generic names for the approved product, but the scientific identifying names used during the trials. The REGN standing for Regeneron. A final generic name that does not reference the inventing company will probably be assigned at some point if this reaches final FDA approval.
[(myl) virtual -> viral fixed — and thanks for the explanation!]
Gregory Kusnick said,
November 13, 2020 @ 10:55 am
So an immunomodulating bamlanivimab inhibitor could legitimately called bamivinalmab?
Scott P. said,
November 13, 2020 @ 11:32 am
In general, we're facing a problem of running out of names for things:
https://harvardlawreview.org/2018/02/are-we-running-out-of-trademarks/
Keith said,
November 13, 2020 @ 12:03 pm
You might find this song amusing (close your ears at around 1 minute and 24 seconds, if you're easily offended by swearing).
https://www.youtube.com/watch?v=ydIKVSMXSlE
Heather Rose Jones said,
November 13, 2020 @ 12:56 pm
A drug from initial development through marketing can have half a dozen different names, from the initial point of "we have no idea if this will pan out so we're just going to give it a number" to what's often a shortened nickname of that once it moves into serious clinical development, to the assigned generic, to the marketing name (which may be different in different markets. And from the inside point of view in the pharmaceutical industry, the really frustrating points are when you have to revise all your documents to use the new name at each stage! When I'm writing up discussions of historic development for one of the drugs I work on, I may have up to five different names for the same product in the references. It can help to think of them as equivalent to registered names versus kennel names!
Vance Koven said,
November 13, 2020 @ 1:49 pm
I wish they had just left it as "bamivimab," so it could be a palindrome.
david said,
November 13, 2020 @ 2:53 pm
@Keith Thanks for the second generation Tom Lehrer litany. I’m surprised that I recognized about 3/4 of them after teaching physiology in medical school, without being a MD.
Philip Taylor said,
November 13, 2020 @ 3:32 pm
Heather — "the really frustrating points are when you have to revise all your documents to use the new name at each stage!". I can strongly recommend the use of TeX — all you need to do is define a macro \thisdrug at the beginning of the document (e.g., \def \thisdrug {Bamlanivimab}), refer to it consistently as \thisdrug {} throughout your document, and then when the drug name mutates you have just one line to change …
Rebecca said,
November 13, 2020 @ 8:53 pm
I was hoping it was stressed like whop bop-a-lu a
Heather Rose Jones said,
November 13, 2020 @ 9:09 pm
@Phillip Taylor – Um…not quite how validated document management systems work in pharma! But hey, thanks for explaining my job to me.
Average Seed said,
November 14, 2020 @ 12:09 am
I am hoping the first three letters were chosen for the knockout effect. BAM! That would take the formation of the name out of the convoluted and into the awesome. I can imagine an Adam West/Batman cartoon style marketing campaign.
Kaleberg said,
November 14, 2020 @ 10:58 pm
If it was named in the US, it was probably Stephanie Shubat and Gail Karet who are responsible for naming generic drugs for the U.S. Adopted Names program at the AMA. I assume they are still working there, though probably from home.
“Sometimes I look at license plates for new prefix ideas,” Shubat confided. “Sometimes I borrow from the names of cats or dogs.”
https://www.latimes.com/business/lazarus/la-fi-lazarus-drug-names-20190719-story.html
As Heather Rose Jones explained, drugs have different names depending on their stage of development. If a drug has a string of digits, odds are it was one of the more promising candidates in a screening effort, one compound among thousands tested for relevant bio-activity. If an illegal version shows up on the street or at a rave, it will probably go by its "party" name and will have a whole list of aliases.